Our Projects
Lipid nanoparticle(LNP)–based mRNA therapeutics are driving remarkable progress in treating both acute and chronic diseases, as well as advancing mRNA vaccines. However, significant challenges still limit their clinical potential—outdated LNP formulations, mRNA degradation, systemic toxicity, insufficient endosomal escape, and off-target delivery all stand in the way of broader application.
Growing evidence points to the need for new strategies that better protect mRNA in biological environments, while also ensuring strong transfection and a robust safety profile for systemic delivery. Our research focuses on tackling some of these fundamental and translational questions.
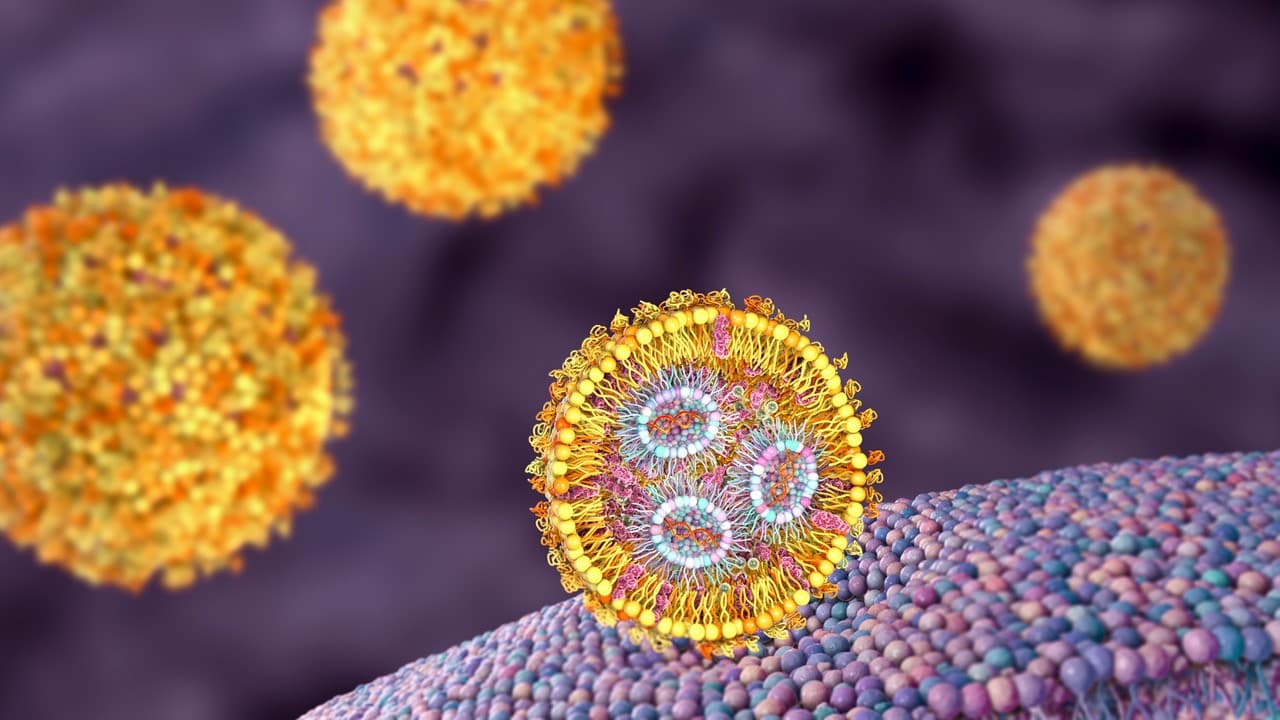
From Barrier to Carrier
By reverse-engineering cell membranes system, we have now been able to encapsulate and deliver nucleic acids. Although this discovery has enabled the delivery of nucleic acids using lipid nanoparticles, one critical challenge remains: genetic cargo often stays trapped inside endosomes instead of being released into the cytosol, forcing the use of higher doses and undermining the safety of mRNA vaccines and gene therapies. Our lab tackles this problem by dissecting endocytosis pathways with state-of-the-art imaging methods—such as super-resolution microscopy and cryogenic electron microscopy—and by creating new lipids and techniques to enable effective endosomal escape.
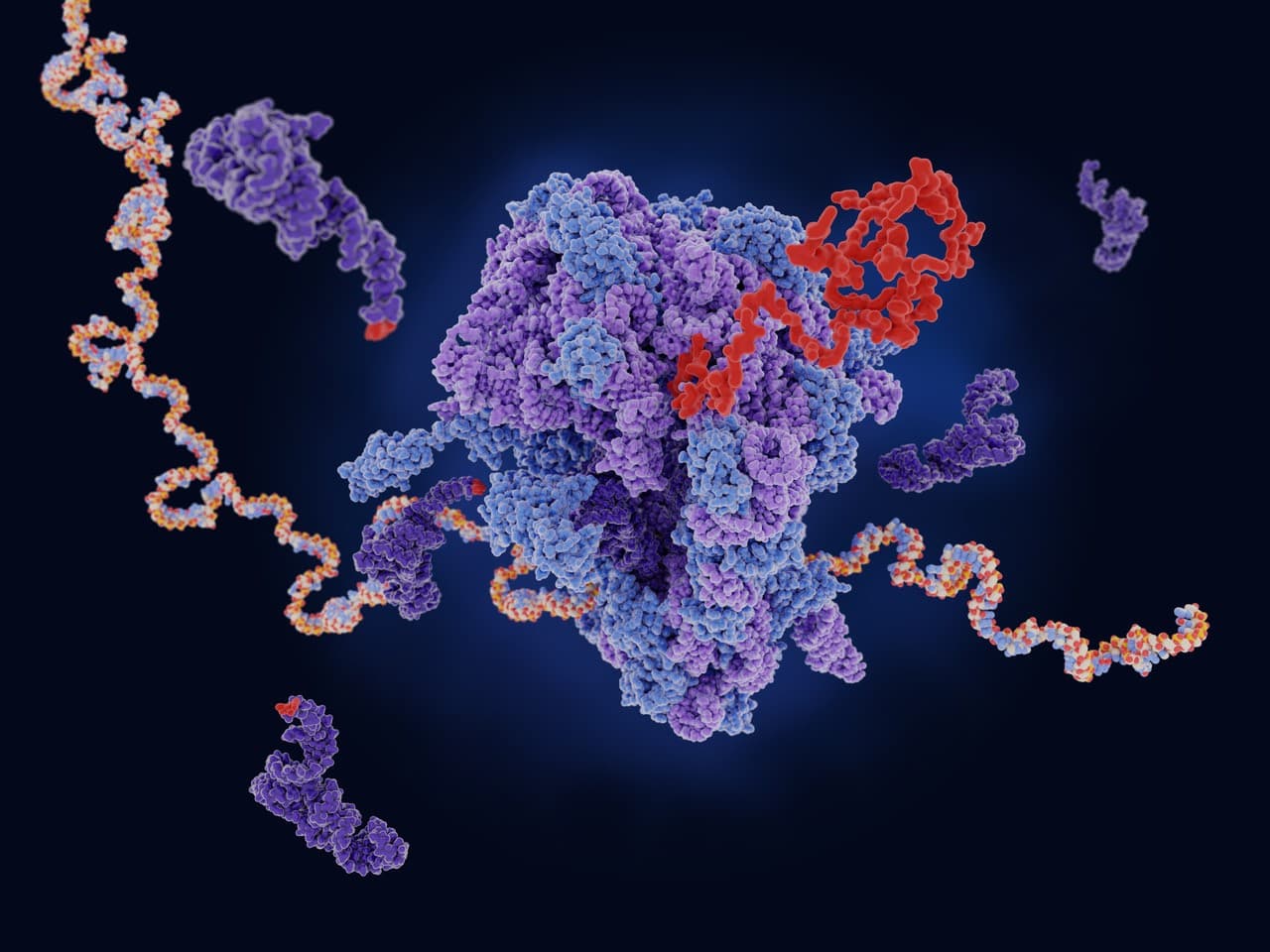
Nanoparticle Structure & Function
LNPs are composed of various lipids, each with distinct functions and properties. These lipid components exhibit a wide range of chemical and structures characteristics, allowing lipids to adapt their geometries and influencing the nanostructure of the formulated LNPs. This, in turn, alters the biophysical properties of the membrane, such as size, surface charge, fluidity, and morphology. These properties plays a significant role in influencing the biological activity and behavior of the LNPs. In our lab, we unravel the structure-activity relationship of LNP through biomembrane studies and formulation approach to develop next-generation LNPs platform and tailored specifically for gene therapy applications as guided by the rational design.
Gene Therapy Applications
Current LNP systems have limited capability for targeted delivery. LNPs are predominantly accumulate in liver and spleen, and limited deposition in extrahepatic tissues. Therefore, the development new activatable delivery strategies would expand the therapeutic applications of LNP-based gene therapy. In our lab, we're working on functional lipids and nucleic acid that can be built into LNPs, including lipid prodrugs and ligand-targeting strategies. We're also developing stimuli-responsive lipid nanoparticles that react to light, sound, irradiation, using porphyrin and porphynoid chemistry, along with bioconjugation techniques.
Research Focus Areas
LNP Formulation Optimization
Developing novel lipid compositions and formulation strategies to enhance delivery efficiency and reduce toxicity.
mRNA Design & Protection
Engineering modified mRNA constructs with enhanced stability and translation efficiency for therapeutic applications.
Targeted Delivery Systems
Creating tissue-specific targeting strategies for precise therapeutic delivery to organs and cell types.
Endosomal Escape Mechanisms
Understanding and improving the release of therapeutic cargo from endosomes into the cytoplasm.
Safety & Immunogenicity
Evaluating and minimizing immune responses while maintaining therapeutic efficacy in clinical applications.
Therapeutic Applications
Translating fundamental discoveries into treatments for cancer, genetic diseases, and infectious diseases.
Porphyrin
Leveraging porphyrin and porphyrinoid chemistry to create stimuli-responsive lipid nanoparticles with advanced functionalization capabilities.
Photodynamic Therapy
Developing light-activated therapeutic delivery systems using photosensitive molecules for precise, controlled treatment activation.